
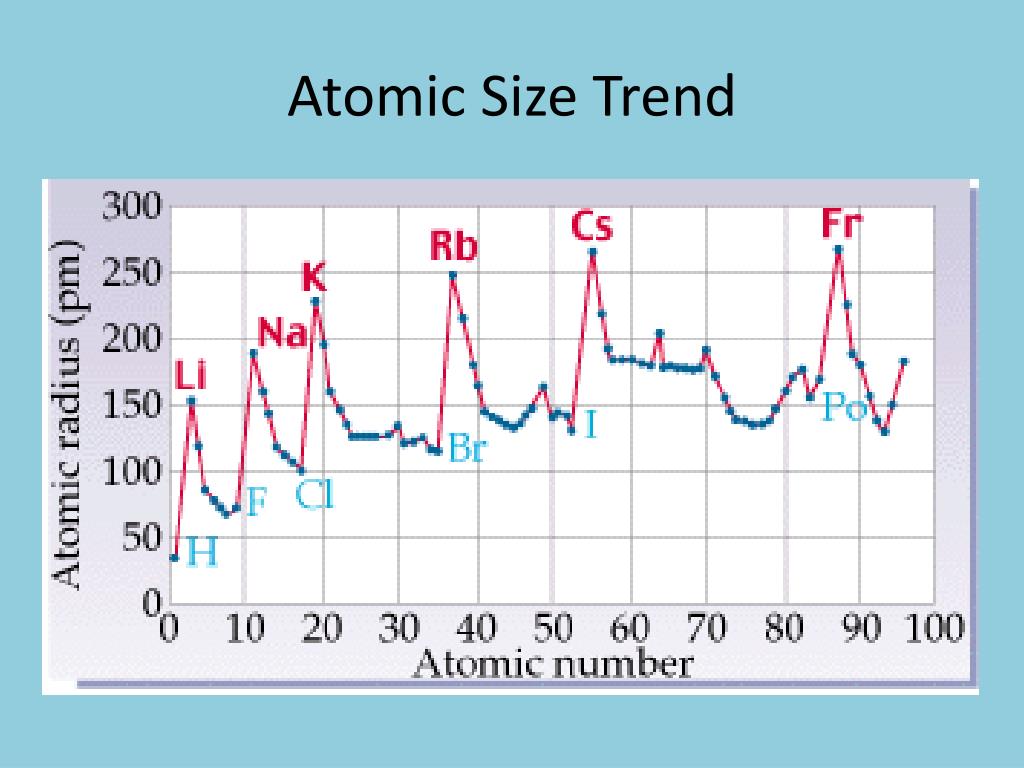
This is also a reason why Li is more reactive than Na. That is why Li has a larger coulombic attraction compared to Na. The charge gets distributed over a larger surface area in Na compared to Li. Na 1 has a bigger atom and more electrons, but the charge is the same as Li 1+. Taking Li 1+ and Na 1+, both have the same charge, but the number of electrons and occupied shells is different. In a charged atom, the bigger the atom, the less is the coulombic attraction. The nucleus is not able to pull the electrons, that are in orbitals further away from the nucleus, towards itself and the coulombic attraction decreases. The bigger the size of the atom, the electrons, especially the valence electrons are further away from the nucleus. The strength of the coulombic attraction depends on two things:

This coulombic attraction causes electrons to orbit around the nucleus.) (The electrons are attracted to the nucleus because the nucleus has positively charged protons in it. positively charged ions and negatively charged ions being attracted to each.protons (which are positively charged) and electrons ( which are negatively charged) attracted to each.Similarly, oppositely charged particles are also attracted to each other. Like, the South Pole of a magnet and the North Pole of another magnet attract. Factors which affect the coulombic attraction Key definition of what Coulombic attraction isĬoulombic Attraction is the attraction between oppositely charged particles.Key points which are covered in this article: Coulombic Attraction in Covalent Bonding.Factors which affect the coulombic attraction.


 0 kommentar(er)
0 kommentar(er)
